 |

For further information, contact the MBL Communications Office at (508) 289-7423 or e-mail us at comm@mbl.edu
FOR IMMEDIATE RELEASE: June 25, 2008
Contact: Carol Schachinger, (508) 289-7149, cschachi@mbl.edu
Genomics of Large Marine Animals Showcased in The Biological Bulletin
MBL, WOODS HOLE, MA—Though the slow moving purple sea urchin may look oblivious, lacking a head, eyes and ears, this prickly creature has an impressive suite of sensory receptors to detect outside signals. And don’t overlook this animal’s self-defense abilities: it has much more ammunition to activate its innate immune system than humans have. The starlet sea anemone lives in coastal areas that face increasing pollution, and it is better equipped than many land, ocean, and freshwater animals to tolerate environmental stress... More>>>
Resources
Photos: Click photos for high-resolution images.

Amphimedon queenslandica, Photo Credit: Bryony Fahey (University of Queensland)
|
 |
 |
|
|
More information on this months' Biological Bulletin cover.
|
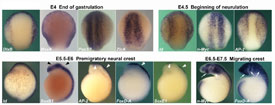
Fig 2
Lamprey
Expression of neural plate border and neural crest specifiers during early neural crest patterning events in lamprey embryos. Dorsal view of E4 and E4.5 lamprey late gastrula/early open neural plate neurula and lateral view of E5.5-E7.5 lamprey neurulae, anterior is to the top. DlxB and MsxA share expression in the ventrolateral ectoderm, but DlxB transcripts are excluded from the neural plate border, whereas MsxA transcripts are present there. ZicA and Pax3/7 transcripts are present throughout the neural plate and its border, where they overlap with MsxA expression.
At E4.5, initial expression of early neural crest specifiers overlaps with that of neural plate border specifiers within the border territory. AP2 expression spans the non-neural ectoderm plus neural plate border; Id is found at lower levels in the non-neural ectoderm, and strongly up-regulated at the border, whereas n-Myc is confined to the open neural plate including neural plate border. AP-2 persists in the premigratory neural crest at E5.5-E6, where it is co-expressed with late neural crest specifiers FoxD-A and SoxE1 (white arrowheads), in a pattern complementary to that of the neural marker SoxB1, which labels the neural tube proper but is excluded from the bulging neural crest (black arrowheads). Id is expressed in the dorsal aspect of the neural tube/prospective neural crest along the entire antero-posterior axis. Both early (n-Myc) and late neural crest specifiers (FoxD-A) are expressed by migrating cephalic neural crest in late neurulae (white arrows).
|
|
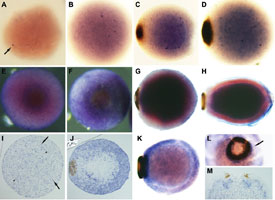
Fig. 3
The demosponge Amphimedon queenslandica
Figure 3. Developmental expression of Amphimedon NK genes during embryogenesis as observed by whole mount in situ hybridization. (A-D) AmqNK2/3/4 (A, B) Cleavage. AmqNK2/3/4 transcripts differentially accumulate in a small number of micromeres (arrow). (C, D) Spot formation/late gastrulation. AmqNK2/3/4-expressing cells are predominantly localised to the inner cell mass; cells with higher levels of expression are localised predominantly on the outer edge of the mass. (E-H) AmqTlx (E, F) Spot stage; posterior view. A high level of AmqTlx expression is present in the cells surrounding and adjacent to the pigment spot. (G) Early ring formation. AmqTlx transcripts are localised in cells of the inner cell mass. (H) Larva. AmqTlx expression remains restricted to the inner cell mass. (I-M) AmqNK5/6/7B (I, J, M) Sections; (K, L) Whole mounts;. (I) Cleavage. AmqNK5/6/7B expression is localised to tiny micromeres (arrow) and slightly larger granular micromeres (arrowhead). (J) Spot stage. These two AmqNK5/6/7B-expresssing cell types are localised to the outer cell region. (K, L) Early ring stage and (M) larva (L, M – posterior view).
|
|